Abstract
Introduction Aggressive hematological malignancies are currently treated with intensive chemotherapy (IC). Despite use of prophylactic antibiotics (ATB), virtually all patients receive wide spectrum antibiotherapy for febrile neutropenia during IC. These treatments induce a strong gut dysbiosis thus disrupting immune homeostasis. Restoring the full gut microbiota ecosystem is a promising therapeutic tool to improve clinical outcomes in patients with acute myeloid leukemia (AML) receiving IC and ATB.
Here we report the tolerability, safety and efficacy of MaaT033, an oral delayed-release capsule containing lyophilized pooled full ecosystem fecal microbiota, in restoring gut microbiota of 21 AML patients having undergone IC and ATB (CIMON: NCT04150393).
Patients and methods 21 patients with AML, receiving IC and eligible to receive consolidation or second induction cycle or allogenic hematopoietic cell transplantation (alloHCT) were distributed in 4 cohorts and treated with escalated dose of MaaT033. For IC, 20 patients received cytarabine associated with an anthracycline, 1 patient received azacytidine. All patients but one received ATB (prophylaxis and/or treatment) during IC and before starting MaaT033 treatment.
Each MaaT033 capsule contains 0.42g of feces i.e a minimum of 109 viable bacteria by pooling fecal material from 4-8 strictly vetted, healthy donors. The dose escalation was performed as follows: 2 capsules 7 days apart for cohort 1 (n=3), 1 capsule/day during 7 days for cohort 2 (n=6), 3 capsules/day during 7 days for cohort 3 (n=6), 3 capsules /day during 14 days for cohort 4 (n=6). 20 patients received the full MaaT033 treatment scheme.
The study primary endpoints included the evaluation of MaaT033 overall safety as well as identification of the adequate dose based on activity on the gut microbiota (engraftment).
Results MaaT033 displayed a good overall safety profile, in line with the profile expected in this patient population.
In total, 5 serious adverse events (SAEs) were reported in 4 patients: 2 in cohort 2 (hyperkalemia due to potassium intake, febrile neutropenia during consolidation), 2 in one patient from cohort 3 (colitis, neutropenic colitis, both during consolidation) and 1 in cohort 4 (infectious diarrhea caused by enteropathogenic Escherichia coli (EPEC) leading to study drug withdrawal). For this SAE, diarrhea started 3 days after MaaT033 treatment start. We did not find the isolated EPEC strain either in the administered MaaT033 batch or in patient's feces before MaaT033 administration by polymerase chain reaction. Absence of pillbox contamination was also verified. Consequently, relationship to MaaT033, although unlikely, could not formally be excluded.
Six infectious events were reported, 1 during MaaT033 treatment phase (SAE described above) and 5 after MaaT033 treatment and during the consolidation cycle (cellulitis, Escherichia sepsis, paronychia, Pseudomonas infection, skin infection, none of them being reported as serious and potentially related to MaaT033).
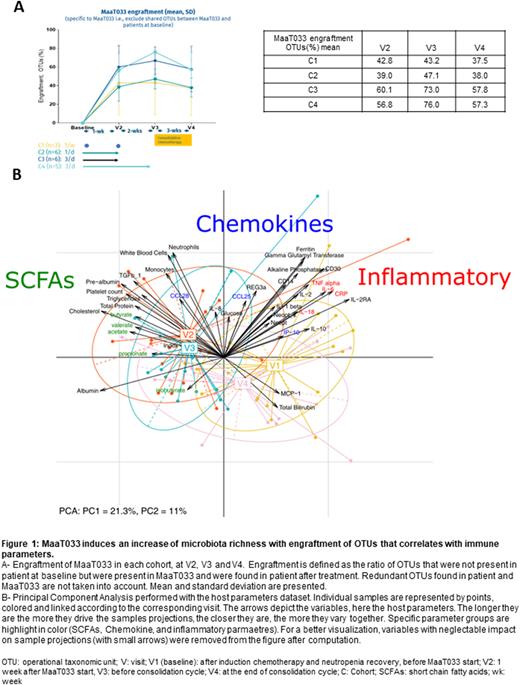
As demonstrated by 16S rDNA sequencing analysis, MaaT033 was able to restore gut microbiota richness at the operational taxonomic unit (OTU) level , and a strong and persistent engraftment of MaaT033 OTUs was shown, especially in cohorts 3 and 4 (Fig1A). MaaT033 bacterial engraftment in patients was inversely correlated with their baseline microbiota richness. Multivariate analysis showed that engraftment correlates with transforming growth factor beta and fecal short chain fatty acids (SCFA) levels and inversely correlates with inflammatory markers such as interleukin 2 and neopterin (Fig1B). At baseline, patients' microbiome composition is associated with relative inflammatory profiles. Following MaaT033 administration (V2/V3), we can observe a switch in patients' microbiome structure, associated with increased fecal SCFA levels, known to have anti-inflammatory properties. After consolidation(V4), the general status of the patients moved to an intermediate state between baseline and V2/V3, which is explained by the systemic and local deleterious effect of the consolidation chemotherapy.
Conclusion The CIMON study results show that MaaT033 is safe, well tolerated and effective for gut microbiota restoration in AML patients receiving IC and ATB. A Phase II/III trial is planned to evaluate MaaT033 in preventing allo-HSCT complications.
Disclosures
Malard:Therakos/Mallinckrodt: Honoraria; Sanofi: Honoraria; Astellas: Honoraria; JAZZ pharmaceuticals: Honoraria; Biocodex: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Gilead: Honoraria; Celgene-BMS: Honoraria. Carre:Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gasc:MaaT Pharma: Current Employment. Jouve:MaaT Pharma: Current Employment. Levast:MaaT Pharma: Current Employment. Plantamura:MaaT Pharma: Current Employment. Prestat:MaaT Pharma: Current Employment. Sabourin:MaaT Pharma: Current Employment. Doré:Biofortis: Honoraria, Membership on an entity's Board of Directors or advisory committees; MaaT Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ysopia: Honoraria, Membership on an entity's Board of Directors or advisory committees; GMT: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novobiome: Membership on an entity's Board of Directors or advisory committees; Nestlé: Consultancy; Eurekare: Consultancy; Enterome: Consultancy. Mohty:Bristol Myers Squibb: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Amgen: Honoraria; Celgene: Honoraria; Astellas: Honoraria; Adaptive Biotechnologies: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria. Recher:AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.